
Upgrade to High-Speed Internet for only ₱1499/month!
Enjoy up to 100 Mbps fiber broadband, perfect for browsing, streaming, and gaming.
Visit Suniway.ph to learn
LOS ANGELES, March 31, 2025 (GLOBE NEWSWIRE) -- Trethera Corporation ("Trethera”), a clinical stage biopharmaceutical company committed to targeting cancer and autoimmune diseases through first-in-class drugs, announced clinical results from treating a patient with ALS (amyotrophic lateral sclerosis, also known as Lou Gehrig's disease) with TRE-515 under the FDA Expanded Access program. TRE-515, a novel drug developed by Trethera and currently in dose escalation trials for solid tumors, targets the enzyme deoxycytidine kinase (dCK) in the nucleoside salvage pathway.
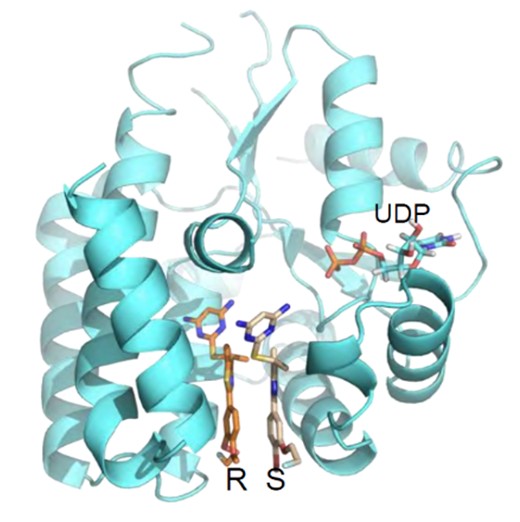
Figure 1. Co-crystal 3D structure of the drug bound to the target enzyme, dCK, at the deoxycytidine binding site.
The patient was diagnosed with ALS in May 2022 and had undergone more than 10 different treatments, including Riluzole, the only FDA-approved drug, without success. In the four months leading up to the trial, the patient's forced vital capacity-a key measure of lung function-declined from 60% to 37%, despite receiving multiple drugs and experimental stem cells to fight the disease. Over a three month dosing period with TRE-515, objective measures demonstrated disease stabilization, including forced vital capacity as well as the Revised ALS Functional Rating Scale (ALSFRS-R). The patient reported gain in body weight, improved neck tone, and increased arm strength over the three month period. Importantly, no adverse events occurred over the treatment duration and the multicenter medical review group voted unanimously to increase the TRE-515 dose and extend the trial.
"Our laboratory research has revealed potential to treat a range of autoimmune and inflammatory diseases, including MS, Crohn's, and lupus. This trial marks a significant milestone as the first clinical use of TRE-515 beyond cancer,” said Dr. Ken Schultz, CEO of Trethera. "Our approach has the potential to extend survival and improve quality of life for patients facing this devastating neurodegenerative disease.”
Get the latest news
delivered to your inbox
Sign up for The Manila Times newsletters
By signing up with an email address, I acknowledge that I have read and agree to the Terms of Service and Privacy Policy.
"FDA approved options to treat ALS are severely limited. Exploring alternatives with first-in-class drugs for patients lacking available therapies represents the continued advancement of science,” said Dr. Frank Diaz, Assistant Professor of Neurology at Cedars-Sinai and treating neurologist. "We are pleased to work closely with Trethera in advancing this novel therapy.”
"We are particularly excited about the potential for TRE-515 as an ALS treatment given its ability to selectively modulate inflammation and its favorable safety profile,” said Dr. Dan Kelly, President of the Pacific Neuroscience Institute Foundation at Saint John's Health Center. "We look forward to continued development of this promising therapeutic candidate for patients in need of therapies that improve the course of disease.”
In collaboration with Cedars-Sinai and Providence Saint John's Health Center, the patient enrolled under the FDA Expanded Access (EA) program. This program enables patients with life-threatening conditions, such as ALS, to access investigational therapies like TRE-515 when no satisfactory alternatives exist. EA trials allow physicians and their patients to explore promising treatments under carefully designed protocols that align with the ongoing clinical development of the drug. When integrated into the drug development process, EA trials offer several benefits - including broader patient engagement, identification of potential response-predictive biomarkers, and valuable data to inform and refine clinical research. However, future randomized clinical trials with larger numbers of patients will be necessary to prove clinical benefit.
About Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease, is a progressive, inflammatory neurodegenerative disease impacting nerve cells in the brain and spinal cord, reducing muscle function and control. ALS can be traced to mutations in more than 20 different genes and is often caused by a combination of multiple subtypes of the condition. Cases cannot usually be predicted, although a small percentage are inherited. ALS has a devastating impact on patients and their families. ALS patients average two to three years of life expectancy after diagnosis and there is currently no cure for the disease.
Sources: Lancet.2022 Oct 15;400(10360); EurJNeurol.2020 Jul 7;27(10); SurgNeuro.2015 Nov 16;6:(171).
About Trethera
Trethera is a clinical stage, privately held, biopharmaceutical company dedicated to pioneering the development of novel treatments for autoimmune diseases and cancers. Founded by prominent UCLA scientists, Trethera is led by experienced management and board members. Trethera's innovative approach to targeting nucleotide metabolism led to the development of TRE-515, an orally administered capsule twice designated by the FDA as an Orphan Drug. TRE-515 is a first-in-class clinical stage drug that inhibits deoxycytidine kinase (dCK), the rate-limiting enzyme in the nucleoside salvage pathway, one of two biosynthetic pathways that generate DNA precursors. It is believed that some forms of cancer may be preferentially dependent on the salvage pathway to support tumor growth, and certain autoimmune diseases might also respond to TRE-515 treatment. Trethera is developing TRE-515 for use as a monotherapy or in combination to precisely target a metabolic vulnerability of cancer or autoimmune diseases that will transform outcomes for patients.
For more information, please visit us at trethera.com or e-mail Investor Relations at [email protected]. You can also follow Trethera on Facebook and LinkedIn.
Note on Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that Trethera believes or anticipates will or may occur in the future are "forward-looking statements,” which may often, but not always, be identified by the use of such words as "may," "might," "will," "will likely result," "would," "should," "estimate," "plan," "project," "forecast," "intend," "expect," "anticipate," "believe," "seek," "continue," "target" or the negative of such terms or other similar expressions. Although Trethera has a reasonable basis for the forward-looking statements contained herein, Trethera cautions that such statements are based on current expectations about future events and are subject to risks, uncertainties and factors relating to medical and scientific research, all of which are difficult to predict and many of which are beyond Trethera's control, that may cause actual results to differ materially from those expressed or implied by the forward-looking statements in this press release. These potential risks and uncertainties include, without limitation: the extent to which development of any novel cancer therapies or therapies for autoimmune diseases succeeds; whether Trethera would obtain the necessary regulatory approvals to commence human trials or commercialize TRE-515 or any novel therapies resulting from such research; Trethera successfully implementing its growth strategy, including that relating to its disease therapies; the effects of the global Covid-19 pandemic; changes in economic conditions; competition; and risks and uncertainties applicable to the business of Trethera. The statements in this press release speak only as of the date hereof and Trethera does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise. The Company intends that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b3b986e5-6dae-4696-9816-43ac934bb36a


 1 day ago
4
1 day ago
4



