Upgrade to High-Speed Internet for only ₱1499/month!
Enjoy up to 100 Mbps fiber broadband, perfect for browsing, streaming, and gaming.
Visit Suniway.ph to learn
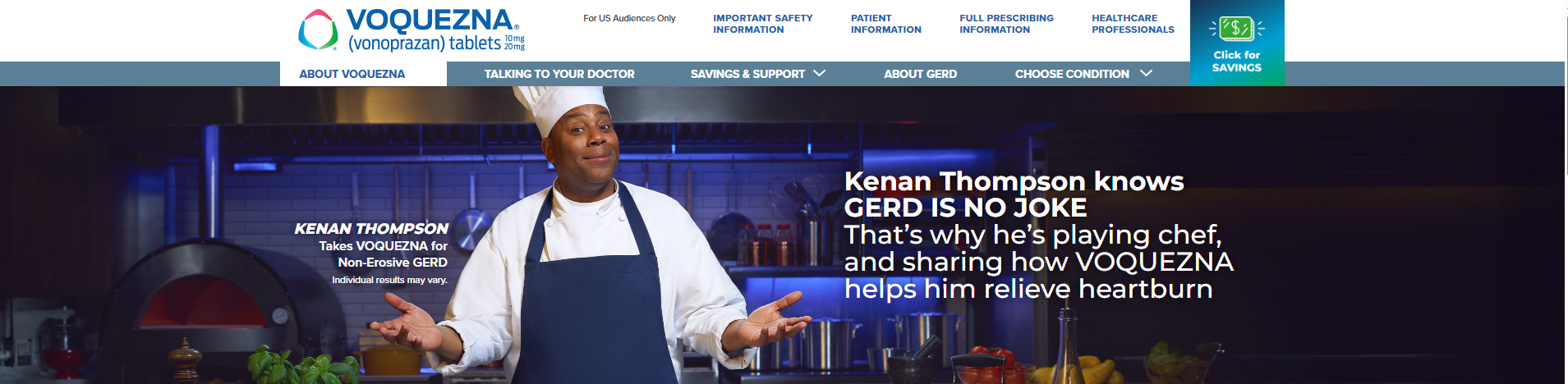
- For the first time, Thompson shares his journey living with gastroesophageal reflux disease (GERD) and experience using VOQUEZNA, as part of the new GERD IS NO JOKE campaign
- VOQUEZNA is a potassium competitive acid blocker (PCAB), the first and only FDA-approved treatment of its kind for both types of GERD1
A Media Snippet accompanying this announcement is available by clicking on this link.
FLORHAM PARK, N.J., March 31, 2025 (GLOBE NEWSWIRE) -- Phathom Pharmaceuticals, Inc. (Nasdaq: PHAT), a biopharmaceutical company focused on developing and commercializing novel treatments for gastrointestinal (GI) diseases, announced today a partnership with actor, comedian, and author Kenan Thompson, best known as the longest-running cast member on Saturday Night Live. For the first time ever, Thompson is publicly speaking out about his experience with GERD as part of the GERD IS NO JOKE campaign to raise awareness about the condition and encourage others to talk with their doctor about treatment options.
Get the latest news
delivered to your inbox
Sign up for The Manila Times newsletters
By signing up with an email address, I acknowledge that I have read and agree to the Terms of Service and Privacy Policy.
Thompson, who has been diagnosed with the most common form of GERD, called Non-Erosive GERD, found heartburn relief with VOQUEZNA® (vonoprazan) after struggling with other treatments. VOQUEZNA is the first and only treatment of its kind approved by the U.S. Food and Drug Administration (FDA) to help manage his condition.1 VOQUEZNA is approved for use in adults for the relief of heartburn associated with Non-Erosive GERD and for the treatment of all severities of Erosive Esophagitis, commonly referred to as Erosive GERD, and relief of related heartburn.1
As one of more than 65 million people in the U.S. living with GERD2-a chronic condition where stomach acid flows into the esophagus, causing irritation and heartburn3-Thompson knows firsthand how disruptive symptoms can be. His goal is to help elevate the discussion around GERD and help others recognize the importance of finding an effective treatment to better manage their disease.
"This is the first time I'm sharing my story with GERD, and trust me-it's no joke,” said Thompson. "I've dealt with terrible burning in my chest, avoided some of my favorite foods, and even had my heartburn affect my voice and sleep, which is tough when your job is to make people laugh. After struggling with treatments that didn't work, my doctor recommended VOQUEZNA, and it helped me kick some acid and reduce my heartburn! I'm excited to partner with Phathom to encourage others to talk to their doctor and find the right treatment for them.”
While Thompson has not experienced side effects while taking VOQUEZNA, some patients taking VOQUEZNA do. The most common side effects of VOQUEZNA for the relief of heartburn related to Non-Erosive GERD include stomach pain, constipation, diarrhea, nausea, and urinary tract infection.1 Please see additional Important Safety Information below.
Thompson shares more about his personal story with GERD at GERDIsNoJoke.com, including a downloadable guide from his own unique point of view, and helpful tips for people to talk to their doctor about the condition.
"Despite using over-the-counter medications and prescription treatments like antacids, histamine-2 receptor antagonists, or proton pump inhibitors-also called PPIs-many people with GERD continue to experience persistent, bothersome symptoms which could affect their daily lives,” said Kavita Kongara, M.D., Motility Clinical Chair and Georgia Physician Executive Committee Member, United Digestive, Physician, Atlanta Gastroenterology Associates. "It's crucial that patients engage in open, honest conversations with their healthcare providers to explore all available therapeutic approaches. VOQUEZNA, a first-in-class PCAB, works differently than traditional medications and provides rapid and durable acid suppression, resulting in effective relief from heartburn and making it an important and differentiated option for GERD management.”
As part of the campaign, Thompson is also taking on the comedic role of a chef in a "Kick Some Acid Cooking Show," a humorous nod to how VOQUEZNA has helped him better control his GERD-related heartburn. This multi-channel campaign will feature a new commercial airing next month on broadcast television and streaming platforms-including Prime Video, Hulu, and Peacock-and will also extend across digital platforms such as Facebook and Instagram, and in waiting rooms at doctors' offices. The goal of the light-hearted campaign is to empower individuals experiencing persistent heartburn to consult their healthcare providers.
"Phathom is passionate about supporting the millions of patients living with GERD and working to advance innovative treatments to address unmet needs," said Martin Gilligan, Chief Commercial Officer at Phathom. "We are excited to partner with Kenan Thompson, who has been making audiences laugh and feel good for decades. Kenan's story is inspiring and relatable to so many GERD sufferers. By sharing his journey for the first time, he's helping to spark conversations and encourage others to talk to their doctors about VOQUEZNA.”
To learn more about Thompson's story, tips for discussing GERD with your healthcare provider, and VOQUEZNA as a treatment option, visit GERDIsNoJoke.com.
A Media Snippet accompanying this announcement is available by clicking on this link.
INDICATION AND IMPORTANT SAFETY INFORMATION
What is VOQUEZNA?
- VOQUEZNA® (vonoprazan) is a prescription medicine used in adults:
- for 8 weeks to heal acid-related damage to the lining of the esophagus (called Erosive Esophagitis) and for relief of heartburn related to Erosive Esophagitis.
- for up to 6 months to maintain healing of Erosive Esophagitis and for relief of heartburn related to Erosive Esophagitis.
- for 4 weeks for relief of heartburn related to gastroesophageal reflux disease (GERD).
It is not known if VOQUEZNA is safe and effective in children.
Do not take VOQUEZNA if you:
- are allergic to vonoprazan or any of the other ingredients in VOQUEZNA. Allergic reaction symptoms may include trouble breathing, rash, itching, and swelling of your face, lips, tongue or throat.
- are taking a medicine that contains rilpivirine (EDURANT, COMPLERA, JULUCA, ODEFSEY) used to treat HIV-1 (Human Immunodeficiency Virus).
Before taking VOQUEZNA, tell your healthcare provider about all your medical conditions, including if you:
- have low magnesium, calcium, or potassium in your blood, or you are taking a medicine to increase urine (diuretic).
- have kidney or liver problems.
- are pregnant, think you may be pregnant, or plan to become pregnant. It is not known if VOQUEZNA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if VOQUEZNA passes into your breast milk. You and your healthcare provider should decide if you will take VOQUEZNA or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
VOQUEZNA may affect how other medicines work, and other medicines may affect how VOQUEZNA works. Especially tell your healthcare provider if you take medicine that contains rilpivirine (EDURANT, COMPLERA, JULUCA, ODEFSEY).
What are the possible side effects of VOQUEZNA?
VOQUEZNA may cause serious side effects including:
- A type of kidney problem (acute tubulointerstitial nephritis): Some people who take VOQUEZNA may develop a kidney problem called acute tubulointerstitial nephritis. Call your healthcare provider right away if you have a decrease in the amount that you urinate or if you notice blood in your urine.
- Diarrhea caused by an infection (Clostridioides difficile) in your intestines: Call your healthcare provider right away if you have watery stools, stomach pain, and fever that does not go away.
- Bone fractures (hip, wrist, or spine): Bone fractures in the hip, wrist, or spine may happen in people who take multiple daily doses of another type of medicine that reduces acid in your stomach known as proton pump inhibitors (PPI medicines) for a long period of time (a year or longer). Tell your healthcare provider if you have a bone fracture, especially in the hip, wrist, or spine.
- Severe skin reactions: VOQUEZNA can cause rare, but severe skin reactions that may affect any part of your body. These serious skin reactions may need to be treated in a hospital and may be life threatening:
- Skin rash which may have blistering, peeling, or bleeding on any part of your skin.
- You may also have fever, chills, body aches, shortness of breath, or enlarged lymph nodes.
If you experience any of these symptoms, stop taking VOQUEZNA and call your healthcare provider right away. These symptoms may be the first sign of a severe skin reaction.
- Low vitamin B-12 levels: VOQUEZNA lowers the amount of acid in your stomach. Stomach acid is needed to absorb Vitamin B12 properly. Tell your healthcare provider if you have symptoms of low vitamin B12 levels, including irregular heartbeat, shortness of breath, lightheadedness, tingling or numbness in the arms or legs, muscle weakness, pale skin, feeling tired, or mood changes. Talk with your healthcare provider about the risk of low vitamin B12 levels if you have been on VOQUEZNA for a long time.
- Low magnesium levels in the body can happen in people who take VOQUEZNA. Tell your healthcare provider right away if you have symptoms of low magnesium levels, including seizures, dizziness, irregular heartbeat, jitteriness, muscle aches or weakness, or spasms of the hands, feet, or voice.
- Stomach growths (fundic gland polyps): A certain type of stomach growth called fundic gland polyps may happen in people who take another type of medicine that reduces acid in your stomach known as proton pump inhibitors (PPI medicines) for a long time. Talk with your healthcare provider about the possibility of fundic gland polyps if you have been on VOQUEZNA for a long time.
The most common side effects of VOQUEZNA for treatment of Erosive Esophagitis and/or relief of heartburn related to gastroesophageal reflux disease include:
| • | stomach inflammation | • | indigestion | ||
| • | diarrhea | • | constipation | ||
| • | stomach bloating | • | high blood pressure | ||
| • | stomach pain | • | urinary tract infection | ||
| • | nausea | ||||
These are not all the possible side effects of VOQUEZNA. For more information, ask your healthcare provider or pharmacist. Call your healthcare provider for medical advice about side effects.
You are encouraged to report suspected adverse reactions by contacting Phathom Pharmaceuticals at 1-888-775-PHAT (7428) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Patient Information and full Prescribing Information for VOQUEZNA.
About Erosive and Non-Erosive Gastroesophageal Reflux Disease (GERD)
Erosive GERD, also referred to as Erosive Esophagitis (EE), is a subtype of gastroesophageal reflux disease (GERD) characterized by erosions in the gastric mucosa caused by acidic reflux of stomach contents into the esophagus.4 There are estimated to be over 65 million individuals with GERD in the U.S.,2 of which approximately 30% have Erosive GERD.3 In addition to experiencing troubling symptoms including heartburn, patients with inadequately treated Erosive GERD may progress to more severe diseases including Barrett's esophagus and esophageal cancer.5
Non-Erosive GERD is the largest category of GERD and is characterized by reflux-related symptoms in the absence of esophageal mucosal erosions.3 It is estimated that approximately 70% of the overall GERD population have Non-Erosive GERD.3 Symptoms of Non-Erosive GERD may impact overall quality of life and can include episodic heartburn, especially at night, regurgitation, problems swallowing, and chest pain.2,3
About VOQUEZNA®
VOQUEZNA® (vonoprazan) tablets contain vonoprazan, an oral small molecule potassium-competitive acid blocker (PCAB). PCABs are a novel class of medicines that block acid secretion in the stomach. VOQUEZNA is approved in the U.S. for the treatment of adults with Erosive Esophagitis, also known as Erosive GERD, the relief of heartburn associated with Erosive GERD, the relief of heartburn associated with Non-Erosive GERD, and for the treatment of H. pylori infection in combination with either amoxicillin or amoxicillin and clarithromycin. Phathom in-licensed the U.S. rights to vonoprazan from Takeda, which markets the product in Japan and numerous other countries in Asia and Latin America.
About Phathom Pharmaceuticals, Inc.
Phathom Pharmaceuticals is a biopharmaceutical company focused on the development and commercialization of novel treatments for gastrointestinal diseases. Phathom has in-licensed the exclusive rights to vonoprazan, a first-in-class potassium-competitive acid blocker (PCAB) that is currently marketed in the United States as VOQUEZNA® (vonoprazan) tablets for the relief of heartburn associated with Non-Erosive GERD in adults, the healing and maintenance of healing of Erosive GERD in adults and relief of associated heartburn, in addition to VOQUEZNA® TRIPLE PAK® (vonoprazan tablets, amoxicillin capsules, clarithromycin tablets) and VOQUEZNA® DUAL PAK® (vonoprazan tablets, amoxicillin capsules) for the treatment of H. pylori infection in adults. For more information about Phathom, visit the company's website at www.phathompharma.com and follow on LinkedIn and X.
Forward-Looking Statements
This press release contains forward-looking statements. Investors are cautioned not to place undue reliance on these forward-looking statements, including statements about the Company's estimates of the number of patients with GERD. The inclusion of forward-looking statements should not be regarded as a representation by Phathom that any of its plans will be achieved. Actual results may differ from those set forth in this press release due to the risks and uncertainties inherent in Phathom's business, including, without limitation: the Company's estimates regarding patient population could prove to be inaccurate and other risks described in the Company's prior press releases and the Company's filings with the Securities and Exchange Commission (SEC), including under the heading "Risk Factors” in the Company's most recent Annual Report on Form 10-K and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Phathom undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
References
- VOQUEZNA. Prescribing Information. Phathom Pharmaceuticals; 2024.
- Machicado JD, Greer JB, Yadav D. (2021) Epidemiology of Gastrointestinal Diseases. In: Pitchumoni CS, Dharmarajan T, (eds). Geriatric Gastroenterology. Springer, Cham. https://doi.org/10.1007/978-3-030-30192-7_7
- Antunes C, Aleem A, Curtis SA. Gastroesophageal reflux disease. NCBI Bookshelf. Accessed January 21, 2024. Available at: https://www.ncbi.nlm.nih.gov/books/NBK441938/?report=printable
- Dickman R, Maradey-Romero C, Gingold-Belfer R, Fass R. Unmet Needs in the Treatment of Gastroesophageal Reflux Disease. J Neurogastroenterol Motil. 2015 Jul 30;21(3):309-19. doi: 10.5056/jnm15105.
- Savarino E, de Bortoli N, De Cassan C, et al. The natural history of gastro-esophageal reflux disease: A comprehensive review. Dis Esophagus. 2017;30(2):1-9.
MEDIA CONTACT
Nick Benedetto
1-877-742-8466
INVESTOR CONTACT
Eric Sciorilli
1-877-742-8466
© 2025 Phathom Pharmaceuticals. All rights reserved. VOQUEZNA, VOQUEZNA TRIPLE PAK, VOQUEZNA DUAL PAK, Phathom Pharmaceuticals, and their respective logos are registered trademarks of Phathom Pharmaceuticals, Inc.


 1 day ago
5
1 day ago
5